As the world shifts to electric vehicles, the demand for resources other than oil and gas has been increasing. One of the key materials required for batteries1 is lithium, which is a soft, silvery alkali metal. The battery of an electric vehicle (EV) requires approximately eight kilograms of lithium metal. You’ve probably heard of mining for other resources (gold, diamond, copper, silver, for example), but the increased demand for lithium has put lithium mining on the minds of battery makers in recent years.
So where does all the lithium come from?
There are two main sources of lithium: rock and brine. For this article, I’ll focus on the latter. Brine is simply water with a high concentration of dissolved salts. The amount of salt that is dissolved in water is measured in “total dissolved solids” or TDS, and the units are typically milligrams per litre (mg/L). Seawater, for example, has a TDS of about 35,000 mg/L. Brines have much higher levels of TDS, over 100,000 mg/L.
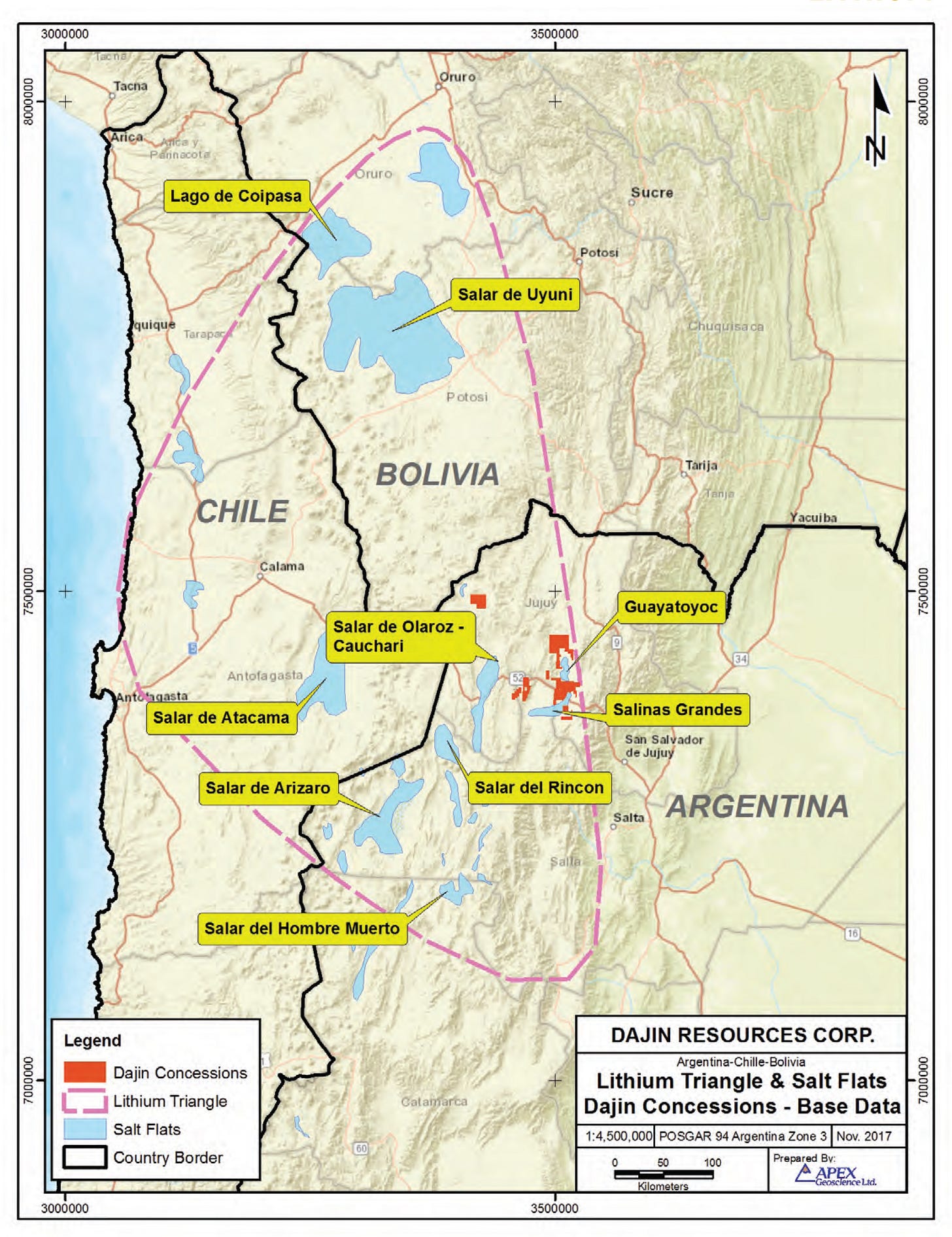
The salts in these brines include sodium, chloride, magnesium, potassium but also can include lithium. Lithium brines exist at different locations around the world, but in South America, there are many dried salt lakes (or “salars”) where brines exist beneath the ground as groundwater. Several of these salars are already producing lithium from the brines and have been doing so for over a decade. In South America, the “Lithium Triangle” covers parts of Chile, Argentina and Bolivia and it’s one of the driest places on earth. The Lithium Triangle in South America is shown in the map below.
The mining process for lithium brines requires pumping of groundwater from a network of wells and releasing the water into evaporation ponds. Because the area gets little precipitation, the water evaporates over a period of months. The salt left behind contains lithium, which is then shipped for processing before ending up in batteries for our EVs, cell phones, laptops etc.
One of the biggest lithium brine mines in the world is the Salar de Atacama in northern Chile. This salar is approximately 100 kilometres long and 80 kilometres wide. This satellite photo shown below is only a small portion of the Salar de Atacama. The rectangular blue/white features are the evaporation ponds. Note the scale of these ponds: close to 10 kilometres long!
The brines at Salar de Atacama have over 300,000 mg/L of TDS and the only way water leaves this salar is by evaporation (i.e., there are no outflows to rivers). Water enters the salar from the surrounding mountains from rain and snowmelt.
The area is very dry and there are no residents on the salar. However, there are small villages around the salar, and along the perimeter of the salar, there are salt-water ponds (or “lagunas”) that are located where the fresh water from the mountains mixes with the brines in the salar. This results in a unique chemistry in the ponds. The ponds provide a nesting ground for flamingos, and it is a tourist attraction.
Mining activities in the salar have had to consider possible impacts on the salars, with questions like: will pumping brine from the middle of the salar affect the water levels or chemistry of the ponds?
A few other points about mining lithium from water:
Technology is improving: The immense evaporation ponds shown above have been the traditional method of extracting lithium from brine. However, researchers are working on direct lithium extraction (DLE) techniques that do not require evaporation ponds.
Other locations: With the advancement of DLE methods for extracting lithium from water, water with lower concentrations2 of lithium, that was previously ignored, is being examined. In western Canada, oil-field brines are under evaluation for lithium. E3 Lithium, a Canadian company is leading the charge at sites in Alberta.
Brines from dried salt lakes have also been mined from a site in Nevada called Silver Peak. This site has produced lithium from brine since the 1960s3 and is currently the only site in the USA that is producing lithium.
In the continued effort to reduce our reliance on fossil fuels, the demand for other resources will inevitably increase. The presence and accessibility of lithium in groundwater has provided an interesting challenge to scientists and engineers who specialize in the study of groundwater (hydrogeology). All types of mining need to consider water and mining lithium from brines is no exception.
In addition to car batteries, lithium is required for batteries in our cell phones, laptops, tablets…
Concentrations in salar brines can be over 1,000 mg/L. In the oil-field brines in Alberta, lithium concentrations are typically under 100 mg/L. The climate of the salars in South America has made evaporation possible, but it is impractical in Alberta where DLE is the only viable solution for lithium extraction from brines.
Besides batteries, lithium is used for ceramics, glass, lubricants as well as in the medical industry.






Learning a ton! Thanks, Steve!